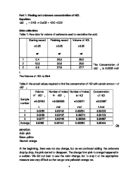
The Concentration of NaOH is 0.0944 mol/ L
The Volume of HCL is 30ml
Table 2: the actual values required to find the concentration of HCl with certain amount of NaOH
Observation:
Base- pink
Acid- transparent
Neutral- transparent
The color of solution, pink, got lighter and lighter as we added the acid. Part 1 and 2 both gave a sudden change, so we carried our experiment very carefully to pour acid drop by drop. With around or slightly over 30㎤, the color of NaOH turned to be very likely to transparent. We found out that a drop would change the color of the solution.
The average concentration of the NaOH solution is 0.0987 ±1.36% M
*note: for the experiment part 1, we used metallic orange as an indicator because we were working with a strong acid and a weak base. On the other hand, we used different indicator in part 2, since we were dealing with a strong acid with strong base.
Processed data:
Equations used
Part 1:
C()= n (CaCO3) / V(solution (Water))
-
Volume of used to neutralize HCl= finishing record – starting record
-
n () =CV
-
n (HCl) = 2 n()
- C (HCl) = n(HCl) / V (HCl)
Part 2:
- Volume of HCl used to neutralize NaOH= finishing record – starting record
- n (HCl) =CV since (=from Part B)
- n (NaOH) = n(HCl)
- C (NaOH) = n(NaOH) / V (NaOH)
V=(litters) n= (mol) C= (mol/L)
Example of calculations:
- Calculation for uncertainties:
Volume of (㎤)
Average= (26.5+ 25.6+ 27.7)/3
=26.6
+) 27.7 – 26.6= 1.1
-) 25.6- 26.6= -1
Uncertainty = sum of the absolute values of + and - /2
|1.1|+|(-1)|/ 2 =1.05
- Volume of neutralizing solution used:
Volume of used (㎤)
Finishing volume – starting volume= 26.9 – 0.4
=26.5
- Concentration of the neutralizing agent:
Concentration of
12.5 g of and 2.5L of water
number of moles in
n= m/M (mol)
= 12.5/ (40.08+ 12.01 + 16.00x3)
=0.125
C= n/V (mol/L)
= 0.125 / 2.35
=0.0535
- Calculating number of moles:
Number of moles in (mol)
26.5㎤ =0.00265L
n=CV
=0.0535 x 0.00311
=0.00142
number of moles in HCl (mol)
n=2()
=2 x 0.00142
=0.00284
- Calculating the concentration of the neutralized agent
Concentration of HCl (L/mol)
C= n/V
= 0.00284. 0.03
=0.09333
- Calculating the percentage of error the final concentration of the solution
% of error of average concentration of HCl (% M)
% of Error = uncertainty/ average concentration x 100
= 0.00367 / 0.0944 x 100
=3.886
=3.89
*note: all other values are calculated in the same way.
Evaluation
Reference:
Comparing the results with another group from classmates;
Group 1
Table 5: the actual values required to find the concentration of HCl with certain amount of
Table 6: the actual values required to find the concentration of HCl with certain amount of NaOH
So, the concentration of NaOH was 0.108.
Group 2
The concentration of NaOH was 0.1101 L/mol.
Average is found by (0.0987+ 0.108 +0.1101) / 3, which is 0.1056, ±5.40% M
Comparing the three results, out final concentration of NaOH is about 6.63% less than the average value. The small difference is made because of uncertainties from each group. From Group 1, I could say that the difference in concentration of HCl is not so big either. So the results are dependant on how accurate that group worked.
Therefore, the value we found for NaOH is appropriate.
Error and Improvements:
Conclusion
From our own experiment, we found that the concentration of NaOH is 0.0987 ±1.36% M when the concentration of nutaralizing agent, HCl, 0.0944 M ±3.89 %.